Properties Of Water
- Alessandro Arda Oriani
- Jan 16, 2017
- 2 min read
Grade 3
Polarity: Water's polarity allows it to dissolve other polar substances easily
Water is called a 'universal solvent' because it dissolves more substances than any other liquid
High surface Tension: because of their polarity molecules are strongly bonded together and this results in a high surface tension
Only substance that can exist in all 3 states: Water is the only substance that can exist in solid, gaseous and liquid states and revert.
High boiling and icing temperatures: water boils at 100( degrees celsius) and freezes at 0 (degrees celsius)
Grade 4
Water is fundamental for life. It makes up 65-75% of our body. Water's ability to dissolve other polar substances guarantees mixture of other substances in our blood. It is very important for life on earth because wherever water goes chemicals, nutrients and minerals are carried, these are all needed to support living things. Another important fact about water is its high surface tension, this allows water to move through plant roots and stems and also the smallest blood cells in our body. This happens because as one molecules moves it pulls the others with it. Water is important in all 3 states. When it is gaseous it is very present in our atmosphere in the form of vapour. The liquid state of water covers 70% of the earths surface and is what we drink. The last advantage water has it that, to differ form other liquid, it doesn't retract when frozen but rather it expands. This means that ice floats on top, which is good as it prevents all life in rivers and ponds to freeze to death.
Grade 5
Glucose: Carried by Blood plasma, due to its polarity it's freely soluble
Amino Acids: soluble in water due to their positive and negative charges. The 'R' group determines their solubility. Like Glucose they are carried by blood plasma
Oxygen: Non Polar. Is soluble due to its small size. As temperature increases so does solubility for oxygen. Hemoglobin in Red Blood Cells carry the oxygen majority
Fats: Insoluble in water. Carried in blood lipoprotein complexes
Cholesterol: Most of the molecules are hydrophobic (away from water) apart from an end which solely composed of hydrophilic (towards water) molecules. Carried in lipoprotein.
Sodium Chloride: freely soluble in water. Carried in blood plasma.
Grade 6 The below table shows a comparison of Methane and water: (http://www.slideshare.net/diverzippy/bioknowledgy-22-water)
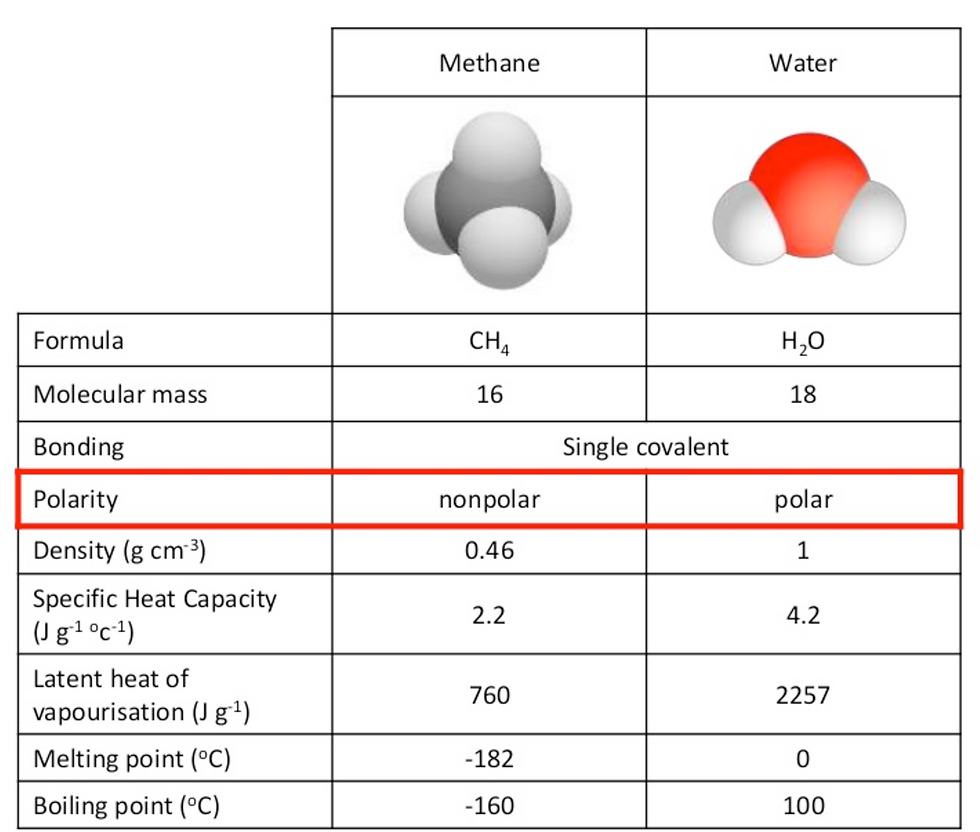
The first argument in favour of water is that its high melting boiling points make it possible for water to exist in many places on earth without evaporating. The second argument is about water's polarity makes it so that it can be used for nearly all reactions. The final argument for water is that its strong density and high surface tension makes it so that it can be compact and sometimes even defy gravity.

Comments